Are you considering PRP therapy, such as Microneedling with PRP? You might have heard a rumor that the FDA banned this treatment, but is that true?
Human platelet-rich plasma (PRP) is beneficial in fast-tracking the healing process due to its high concentration of potent growth factors.
So, what is the FDA’s official stance on PRP therapy?
What Is the Collection Method for Harvesting Platelet-Rich Plasma?
Before we get to the FDA, let’s unpack the specifics behind PRP to give you an understanding of the dynamics of the therapy. First, it’s important to note that PRP is already a successful treatment, with a legacy extending back some two decades.
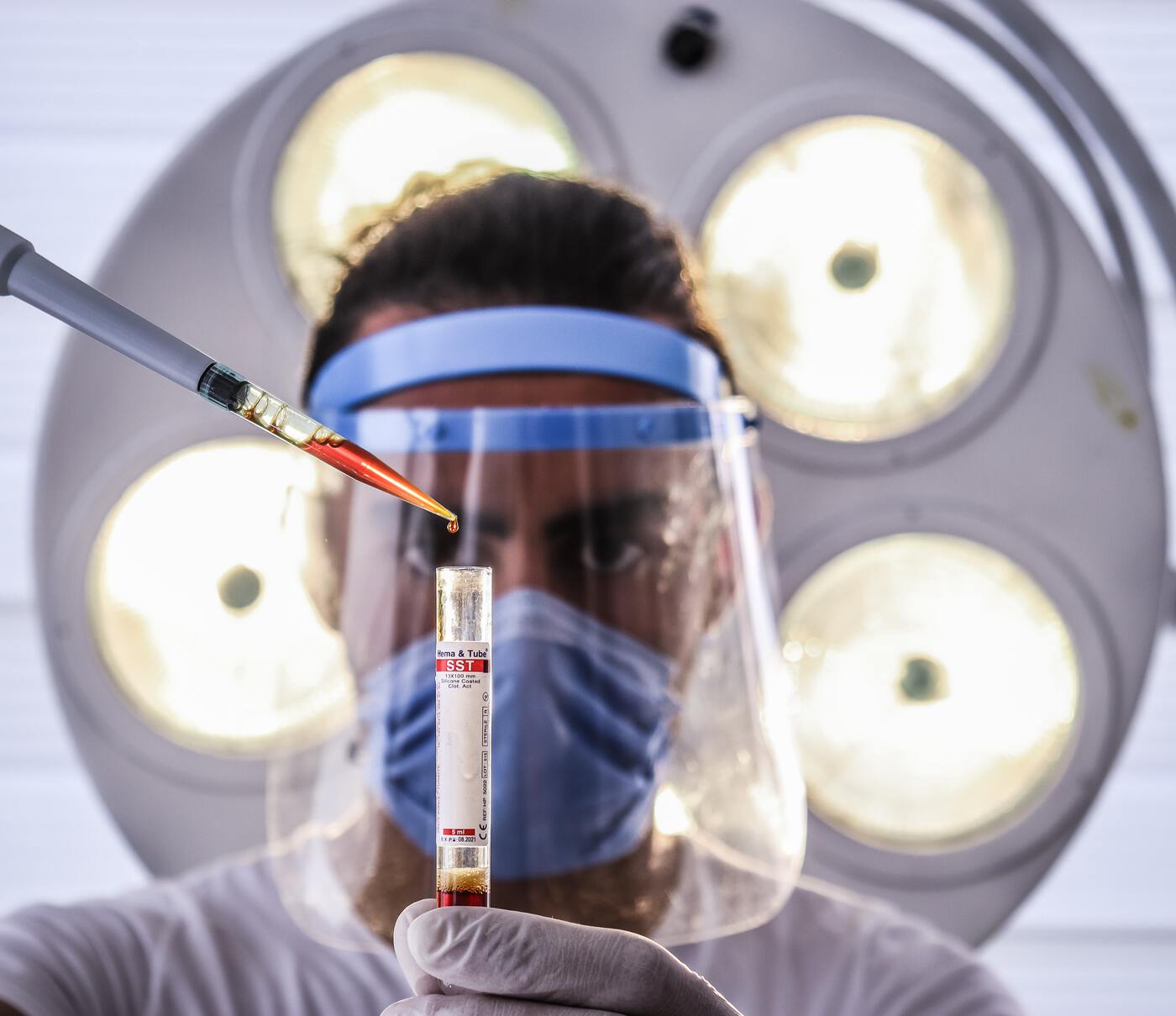
This medical procedure utilizes human platelet-laden plasma separated from whole blood cultures. Qualified technicians employ the therapy to treat a sports-related injury, various chronic problems, and musculoskeletal issues.
Technicians remove whole blood cells from patients, sending them to a centrifuge to separate the platelet-rich plasma. There are two methods to the process: “Buffy Coat” and “PRP.”
Both processes are similar, combining whole blood cells with anti-coagulants before adding them to the centrifuge.
What are the Benefits of PRP Therapy?
Healing Musculoskeletal Injuries
Orthopedic sports medicine relies on PRP for fast-tracking healing of torn muscles and ligaments and skeletal injuries to joints.
Healing Nerve Injuries
PRP can help resolve neuromuscular disorders and injuries to the nervous system, such as peripheral neuropathy.
Cosmetic Treatments
There is evidence that PRP treatments are effective in anti-aging therapies, delivered via dermal injection or skin care treatments such as SkinPen Microneedling. PRP therapy can reduce wrinkles in the face, hands, and neck, providing a more youthful appearance to the patient.
PRP treatments can also assist with accelerating healing times after cosmetic procedures like hair transplants.
Is PRP Banned by the FDA?
The FDA is closely involved with the approval of all biologic treatments involving the use of devices in the procedure. The FDA has an active role in clearing and approving all PRP devices and treatments.
Recently there have been concerns with a certain FDA letter regarding tissue based biologics: Framework for the Regulation of Regenerative Medicine Products’, 21CFR Part 1271 regulation of human cellular tissue/ products (HCT/P).
The FDA guidance and 21CFR Part 1271 do not apply to the use of PRP as it is considered a blood component, not a human cellular or tissue product.
Medical Spa industry association AmSpa’s Legal Coordinator Patrick O’Brein states in response to this topic:
The FDA’s framework for regenerative medicine products using human cells or tissues (referred to as HCT/P) has very specific definitions regarding which type of tissues or products qualify as a HCT/P. While the FDA may change its guidance in the future, PRP that is derived from the patient’s own blood (such as through a centrifuge) is currently not considered an HCT/P; instead, products and treatments that use stem cells, liquids and other substances extracted from other tissue—such as adipose fat or placental/umbilical cord blood—do fall under these rules
You’ll find it comforting to know that the FDA approved both the Buffy Coat and PRP methods in 2009. So, yes, PRP treatments are FDA approved.
Right now, there are several dozen devices on the market used in PRP treatments. The vast majority of these devices do have FDA approval. However, there are some, especially from imported sources, that don’t have FDA approval. In fact, these imported devices may not even be on the FDA’s radar yet.
PRP treatments themselves use a patient’s blood. Therefore, they are not officially “drugs” in the eyes of the FDA. Consequently, they are not required to have any approval for use.
Before you book a PRP treatment, you’ll need to understand treatments and procedures widely used in a clinical setting, don’t have FDA, but have registration as “cleared” for use by the FDA.
The is no official approval from the FDA regarding the use of PRP in treatments for sports-related injuries. However, despite the lack of regulation, it’s one of the most popular regenerative therapies used by athletes across all sports.
PRP offers the athlete the opportunity to fast-track healing times, getting them back to training and competition faster than a conventional rehabilitation program.
As of 2021, FDA approval for PRP injection therapies in the treatment of tendonitis is still under review by the FDA.
The FDA has issued approval for PRP therapies in the treatment of diabetic ulcers in affected individuals. There are also instances of FDA approval for use in the acceleration of healing from orthopedic surgery.
So, with so many treatments still pending FDA approval, what’s the final verdict on FDA approval for PRP therapies?
It looks like the FDA’s bottom line on the use of PRP therapy seems to be to approve it for use in enhancing recovery from medical and surgical procedures. It’s for this reason that both treatments and devices used in PRP are “cleared” by the FDA instead of “FDA-approved.”
In Closing – PRP into the Future
Since PRP is a natural preparation sourced from the human body, we expect that the FDA will continue its run of approving all PRP devices and treatments. There are still many therapies and devices under review or not under review by the FDA.
We expect it’s going to take a few years for the organization to get round to recognizing and classifying all PRP therapies and devices.
However, it’s important to note that as of June 21st, 2021, all distributors of PRP devices require a valid biologics license from the FDA.
Those distributors currently in a prototype development stage must understand that their device may only undergo human use in an FDA-sanctioned clinical trial with approved IND.
All medical devices only have approval for use within specific parameters set by the FDA and the manufacturer. The off-label use of a PRP device without IND approval or a specific medical device exemption is prohibited.